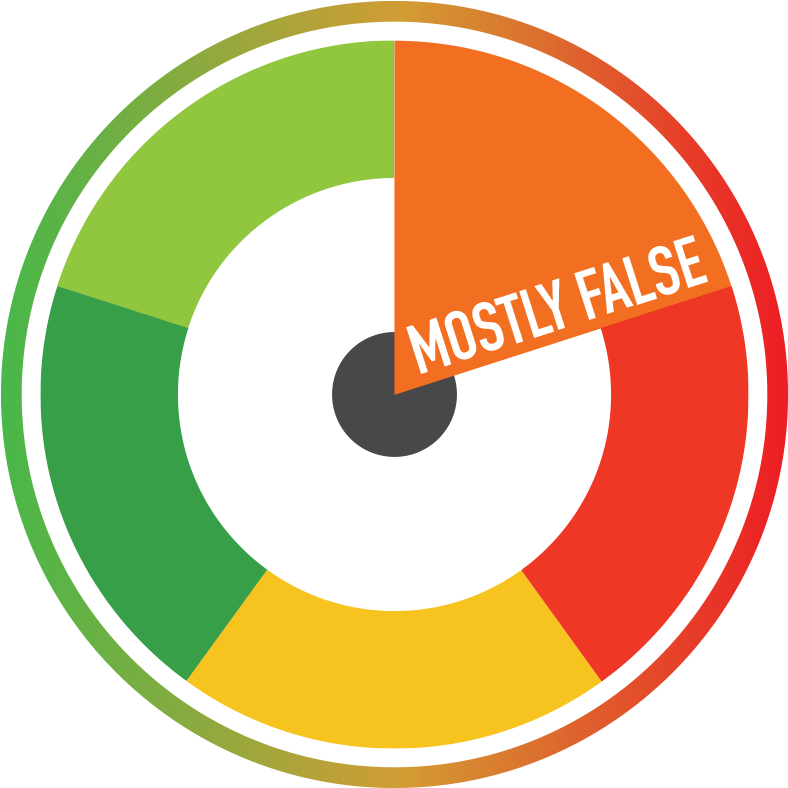
Italian Prime Minister Giuseppe Conte announced that the anti-Covid drug will begin to be distributed in just over a month: “If the final stages of preparation of the Oxford-Irbm Pomezia-AstraZeneca vaccine will be completed in the coming weeks, the first doses will be available in early December. The statement, taken from the book “Why Italy loved Mussolini (and how he resisted Covid’s dictatorship)” by journalist Bruno Vespa, was made public on 20th October 2020.
The Italian Prime Minister’s statement has served to temper the anger of the workers most affected by the pandemic, but it does not fully correspond to reality: in fact, experimental studies on the drug should be completed between the end of November and the first weeks of December, while the actual distribution of the doses will not begin before Christmas or January 2021.
This was confirmed by Piero Di Lorenzo, CEO and Chairman of IrbmPomezia, an Italian company working on the development of the vaccine with researchers at Oxford University and the pharmaceutical company AstraZeneca. According to his statement “it is reasonably credible that around 3 million doses will arrive in the country by the end of 2020”. Before that, he specified, “production has been going on for a couple of months. The hope is that the vaccine doses will be available by the end of the year. Let’s keep our fingers crossed”.
The gap between the hypothesis suggested by PM Conte and the wish of the scientists is significant. If the last few weeks of experimentation were not to continue as expected, the gap would increase.
The clarifications – relaunched by many British newspapers, including the Daily Mail – by professor Adrian Hill, head of the vaccine research project at Oxford University, also confirm the timing announced by the Italian partners. In addition, the drug has to be approved by the European Medicines Agency (Ema) before it is officially placed on the market and this will only take place at the beginning of 2021, with mass distribution in late spring.
Ema’s own website explains that the time required for the analysis and approval of the vaccine is shortening compared to the normal guidelines. The development of a new vaccine usually takes up to ten years: this is the case with the research that is still being carried out on the Ebola virus. The mass mobilisation that has taken place to try to curb the new pandemic is ensuring that everything behind the marketing of a vaccine – from the analysis of preliminary results to the bureaucracy that regulates the approval of a drug under European Regulation 726/2004 – is completed in a maximum of two years. This is shown by the fact that the Ema is committed to analyze the data provided by Oxford about every fortnight in twenty days compared to the usual 40-70 and that it takes decisions on the approval of the different steps in 48 hours instead of 10 days. The same acceleration will also happen for the final OK before the marketing of the vaccine (from 210 days to 150), with the promise to do everything possible to further speed up the bureaucratic procedures.
As reported by the World Health Organization, there are three stages in the preparation of a vaccine. In the case of the Oxford drug, the first part of the trial involved 1077 participants: Only one part received the drug, while the other was treated with placebo. Volunteers only knew what had been given to them at the end, so researchers were able to isolate any false positives.
The next two phases involved 10,560 people across the UK, including adults aged between 56 and 69, over 70 and children aged 5-12. The main reason for the drug’s effectiveness is the immune response in the elderly, confirmed by Oxford on 26 October.
“If everything goes smoothly we will be able to authorise the first vaccines between January and February”: this is what Guido Rasi, Ema’s Executive Director for nine years, said on the pages of Italian daily La Repubblica on 28 October. It is “extremely difficult if not unlikely” that a vaccine could arrive before Christmas, as announced by PM Giuseppe Conte. Moreover, it is necessary to understand that the first doses will serve to “vaccinate immediately the categories at risk so that by the summer we can have enough vaccinated to see the effects on the pandemic”.
The battle against Covid-19 is still long and exhausting because, concludes Ema number 1, to make the whole of Europe immune “you need 500-600 million doses and having them by the end of next year will not be possible”.
If someone therefore thought he was in the race for the vaccine, he will not find satisfaction: at least at the beginning the drug will be administered – still partially experimental – giving priority to medical staff, the elderly and people with other serious diseases. To be really sure of keeping the pandemic under control, however, it will take the end of 2021.
RESEARCH | ARTICLE © Viviana Astazi, Luigi Scarano, Pasquale Ancona, Davide Cavalleri, Francesca Canto
Leave your comments, thoughts and suggestions in the box below. Take note: your response is moderated.